Introduction
Substantial progress in the treatment of Multiple Myeloma (MM) extends survival for many patients (Pts), even after multiple relapses, owing to a broad and rapidly extending therapeutic arsenal, with major advances in the arena of bispecific antibodies and CART therapy. However, this also creates an increasingly complex challenge in selecting optimal therapeutic modalities for each patient, as current guidelines rely on empirical considerations. To address this, our study employs a population-scale single cell genomics database of over 250 patient samples covering all disease stages and cycles of treatment with complete patient medical records and machine learning approaches to analyze molecular signatures of myeloma cells and bone marrow immune microenvironment.
Methods
This study constitutes a meta-trial of RRMM patients who underwent single-cell RNA sequencing (scRNA-seq) between 6.2017 and 6.2023 in our lab. We gathered comprehensive demographic and myeloma clinical data, with a particular focus on therapeutic history and drug refractoriness, defined based on IMWG criteria (progression on or within 60 days from treatment). Our clinical scRNA-seq pipeline, includes a tailored protocol involving index sorting of CD38+ CD138+ positive cells for myeloma plasma cells (PCs) (Ledergor et al, Nat Med 2018; Cohen et al Nat Med 2020), together with CD45+ sorting and scRNA-seq cells from the tumor microenvironment for to identify tumor -immune molecular signatures associated with drug resistance.
Results
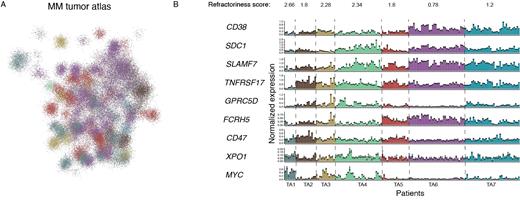
A total of 243 patient samples were included in the study, comprising RRMM (N=128), NDMM (N=21), SMM (N=9), and MGUS (N=4) patients with clinical annotation. Among RRMM patients, the median age was 72 (range 40-89), and 59% exhibited high-risk FISH cytogenetics (t(4:14), del17p, t(14:16), t(14:20), +1q21), while 17% had double-hit myeloma. Refractoriness to specific drugs was observed, such as bortezomib (49%), carfilzomib (27%), lenalidomide (49%), pomalidomide (33%), daratumumab (49). In total, we sequenced 158,444 myeloma/plasma cells, with an average of 880 cells per patient, along with 800 CD45+ cells. By using plasma cells from 11 healthy donors as a reference, we calculated transcriptional changes for each patient, determining the upregulated and downregulated genes profile. Clustering the patient's tumor profiles revealed 7 main tumor archetypes [TA] defined by the expression of 8 gene modules.
Refractoriness score, defined as the average number of drugs to which myeloma is refractory, exhibited variations across different tumor archetypes. Notably, the expression levels of several tumor drivers and crucial targets of novel immunotherapies also showed diversity among the tumor archetypes. For instance, TA#6, primarily comprising newly diagnosed patients, displayed lower expression of GPRC5D. Conversely, in TA#1, which consisted of ultra-refractory patients with high MYC and CD47 expression, both GPRC5D and TNFRSF17 (BCMA) were downregulated. Examination of bone marrow CD45+ immune cells revealed a striking bimodal immune composition, characterized by one subtype with increased T-cell abundance and another enriched with myeloid cells. Each immune cell subtype contributed to the drug responsiveness score and provided additional insights complementary to the tumor cells' profile. Further clinical and transcriptional correlations involving both MM cells and immune cells will be presented at the meeting.
Conclusions
We constructed a clinically-annotated transcriptional matrix revealing the blueprint of relapsed refractory myeloma. This novel decision engine provides a basis for optimizing treatment selection by considering both tumor and tumor microenvironment characteristics. By incorporating critical information on drug resistance patterns and expression of key targets like GPRC5D, TNFRSF17 (BCMA) and FcRH5, we enable precision medicine approaches for improved management of relapsed refractory myeloma. These findings open avenues for tailored and more effective therapeutic strategies, promising better outcomes for patients with relapsed refractory myeloma.
Disclosures
Cohen:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Avivi Mazza:AbbVie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal